Calculate ∆G° for the following reactions at 25° C:
(a) N2 (g) + O2 (g) → 2 NO (g)
(b) H2O (l) → H2O (g)
(c) 2 C2H2 (g) + 5 O2 (g) → 4CO2 (g) + 2H2O (l)
Standard free energy formation values:
| Standard free energy formation (ΔG°f) | kJ/mol |
| N2 (g) | 0 |
| O2 (g) | 0 |
| NO (g) | 86.7 |
| H2O (l) | -237.2 |
| H2O (g) | -228.6 |
| C2H2 (g) | 209.2 |
| CO2(g) | -394.4 |
Interpretation
To express the spontaneity of a reaction more clearly, we introduce thermodynamics’ function called Free energy or Gibb’s free energy (G). It is the energy available in the system to do useful work.
Gibb’s free energy is defined as a thermodynamic equation equal to the enthalpy of a system, minus the product of the entropy and the temperature of the system.
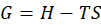
Where, G = Gibb’s free energy
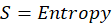
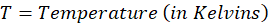
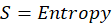
The value of Gibb’s free energy (G) is expressed in Joules or Kilojoules
Formula
We can calculate standard free energy change by using the following formula:
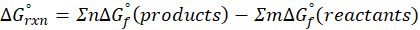
Where,
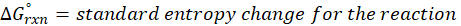
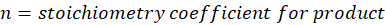
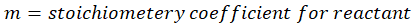

Solution:
(a) N2 (g) + O2 (g) → 2 NO (g)
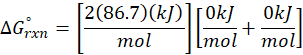
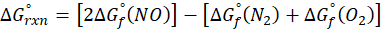
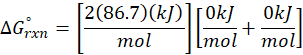
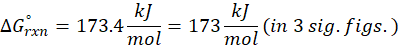
Therefore, ∆G°rxn for the reaction N2 (g) + O2 (g) → 2 NO (g) is 173.4 kJ/mol.
(b) H2O (l) → H2O (g)
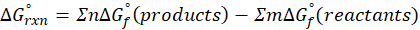
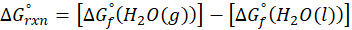
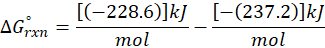
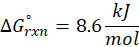
Therefore, ∆G°rxn for the reaction H2O (l) → H2O (g) is 8.6 kJ/mol.
(c) 2 C2H2 (g) + 5 O2 (g) → 4CO2 (g) + 2H2O (l)
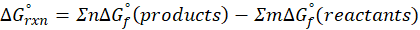
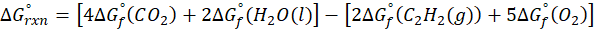
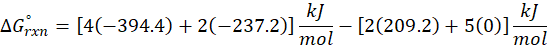
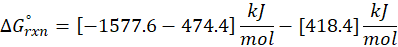
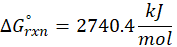
Therefore, ∆G°rxn for the reaction 2 C2H2 (g) + 5 O2 (g) → 4CO2 (g) + 2H2O (l) is 2740.4 kJ/mol.
