Determine whether the entropy change is positive or negative for each of the following reactions and explain the reasoning behind your predictions.
- 2 KClO4 (s) → 2 KClO3 (s) + O2 (g)
- H2O (g) → H2O (l)
- 2 Na (s) + 2 H2O (l) → 2 NaOH (aq) + H2 (g)
- N2 (g) → 2 N (g)
Interpretation:
Entropy is a measure of the randomness or disorder within a system. The higher the degree of randomness, the greater the entropy. Among the three primary states of matter, gases possess the highest entropy. The vapor state offers more space for molecules to move compared to the liquid state, and the liquid state provides more freedom of movement than the solid state. Water molecules are ordered more in the solid state than in the liquid or gaseous states. Hence, the entropy order is as follows: Ssolid < Sliquid < Sgas .
Here, we aim to determine whether the entropy change is positive or negative in the context of the given reactions. To do this, we will examine the states of the reactants and products involved in the chemical reaction. If the number of gas molecules (∆ng) increases during the reaction, it results in an increase in entropy, indicating a positive entropy change. Conversely, if the number of gas molecules decreases, the entropy change will be negative.
To calculate this, we will apply the appropriate formula.
Formula

(If ∆ng is positive, then entropy change will also be positive)
1. 2 KClO4 (s) → 2 KClO3 (s) + O2 (g)

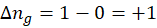
∆ng is positive, so the entropy change will be positive. Entropy will increase as more and more gas is created.
2. H2O (g) → H2O (l)
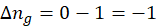
∆ng is negative, so entropy change will be negative. The formation of liquid will decrease entropy as randomness decreases.
3. 2 Na (s) + 2 H2O (l) → 2 NaOH (aq) + H2 (g)
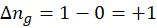
∆ng is positive, so entropy change will be positive. The entropy will increase as more gaseous products are formed.
4. N2 (g) → 2 N (g)
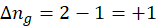
∆ng is positive, so the entropy change will be positive. The entropy will increase as more and more gas is created.
