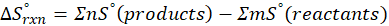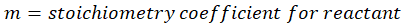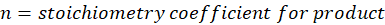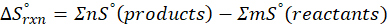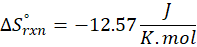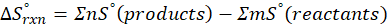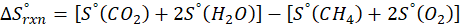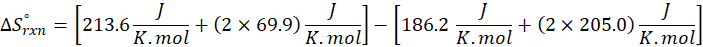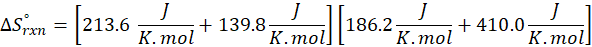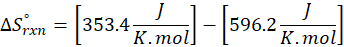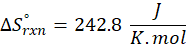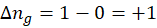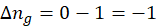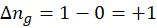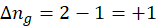How does the entropy of a system change in each of the following processes?
- Melting of solid
- Freezing of liquid
- Boiling of liquid
- Conversion of vapor to solid
- Condensation of vapor to liquid
- Sublimation of solid
- Dissolution of urea in water
Entropy
In Thermochemistry, entropy is the degree of randomness or disorder in a system. The greater the degree of randomness, the higher the entropy, and vice versa. Entropy describes the spontaneous changes that occur in everyday life or the tendency of the universe towards disorder.
For example, In the morning, when you clean your room and arrange everything neatly, the system has a higher order, however, as you begin your daily activities, especially cooking, things get messy. This is an example of how entropy initially appears lower in an organized state, but as disorder increases and things become more chaotic, the entropy increases.
Entropy is a thermodynamic function that depends on the system’s state rather than the pathway followed. Entropy is an extensive property that scales with the system’s size or extent.
Interpretation
When we increase the temperature, entropy increases. Adding more energy to a system causes the molecules to become more excited, leading to greater randomness. The more random the system, the higher the entropy. Now, let’s determine the change in entropy for the following scenarios:
a. Melting of solid:
In its solid state, ice has molecules fixed in an ordered structure. As it begins to melt, the molecules gain mobility, leading to increased disorder and consequently greater randomness. A higher degree of randomness corresponds to higher entropy. Since the liquid state is more disordered than the solid state, the system’s entropy increases when ice melts.
b. Freezing of liquid
When a liquid freezes, its molecules become more ordered, resulting in a solid with a fixed structure and less randomness. As a result, the degree of disorder decreases. Hence, entropy decreases during the freezing process of liquid.
c. Boiling of liquid
When a liquid begins to boil, the molecules gain more freedom to move independently, which leads to an increase in randomness. The more random a system, the higher its entropy. Since the gaseous state is more random than the liquid state, entropy increases when the liquid turns into gas.
d. Conversion of vapor to a solid
When vapor turns into a solid, the molecules have less freedom to move, causing the level of randomness to decrease. As a result, entropy decreases because the water molecules are more organized in the solid state than the vapor state.
e. Condensation of vapor to liquid
Vapor molecules have more free space to move compared to liquid molecules. Molecules become more organized when the vapor turns into liquid and the randomness decreases. Therefore entropy decreases.
f. Sublimation of solid
When solid sublimes, it is converted to vapor state. As molecules are more ordered in the solid state than the gaseous state, the randomness in the gaseous state is greater, and therefore entropy increases.
g. Dissolution of urea in water
When urea dissolves in water, it forms solid crystals or pellets and is highly soluble. The process of dissolving urea involves transitioning from a more ordered solid state to a less ordered liquid state. As the urea dissolves, the randomness increases, resulting in increased entropy.