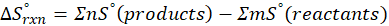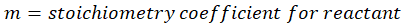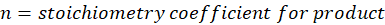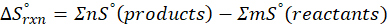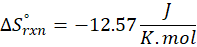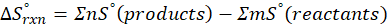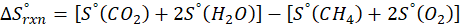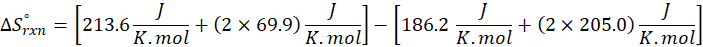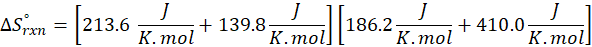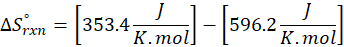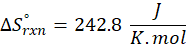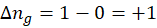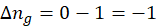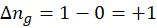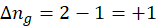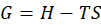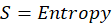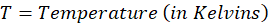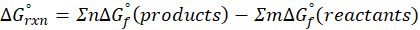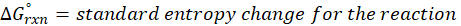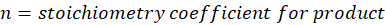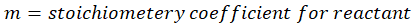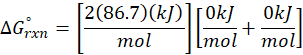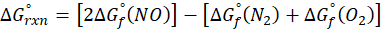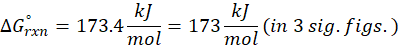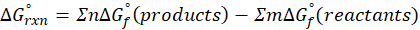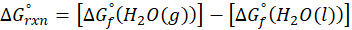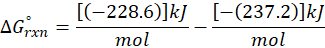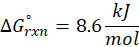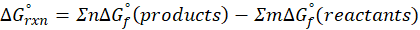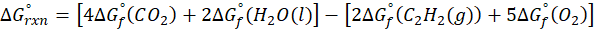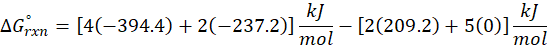Thermochemistry is the branch of chemistry in which we study the heat energy involved in chemical reactions and phase changes, such as melting and boiling. For example, adding heat to ice can change its state from solid to liquid.
Thermochemistry helps us explain how much heat is released or absorbed quantitatively.
Thermochemical reactions are classified into two categories:
- Exothermic Process, and
- Endothermic Process
1. Exothermic Process:
In an exothermic process, the system releases heat energy into the surroundings, resulting in an increase in the surrounding temperature. This release of energy illustrates an exothermic process.
Example:
- Making ice cubes: As the temperature of the water decreases and it transitions from liquid to solid, it releases heat into the surroundings. This release of heat characterizes exothermic reactions, where the system releases energy rather than absorbing it.
- Mixing water and strong acid: When we mix acid into water, they react vigorously, releasing heat energy into the surroundings. This release of heat conveys an exothermic reaction.
Note: Always add acid to water, not the other way around, as adding water to acid can cause it to splash or erupt violently.
2. Endothermic Process
In an Endothermic process, the system absorbs heat energy from the surroundings, thus decreasing the surrounding temperature.
Example:
- Cooking an egg: Egg absorbs heat energy from the pan or water, causing changes to its internal structure. This transformation is what cooks the egg and is characteristic of an endothermic process, where the system absorbs energy.
- Melting ice cubes: During melting, ice absorbs heat from the surroundings, which is characteristic of an endothermic process. In this process, the temperature of the ice stays constant at the melting point (0° C ) during the phase change from solid (Ice) to liquid(water). The temperature will only increase when the ice completely melts and we continue adding heat.
Enthalpy of reaction: Enthalpy is used to measure the energy in a system.
When a chemical reaction is given, we can find out the change in enthalpy by the following formula:
ΔHrxn = ∑ΔHproducts – ∑ΔHreactants
Where,
ΔHproducts = Sum of total enthalpy absorbed/released by the products
ΔHreactants = Sum of total enthalpy absorbed/released by the reactants
We can use the above formula to identify whether the reaction is exothermic or endothermic. If ΔH reaction is positive, the reaction will be endothermic, and if ΔH is negative, the reaction will be exothermic.
Energy
Energy is the capacity to do work. In thermochemistry, we prioritize heat energy (the heat exchange between a system and its surroundings during phase change and chemical reactions).
Energy Transfer
As the term suggests, energy transfer refers to the movement of energy from one system or object to another. In thermochemistry, energy transfer specifically refers to the flow of energy, primarily in the form of heat or work, due to differences in temperature, pressure, or other conditions.
Latent heat
Latent heat is the heat energy necessary to change the phase of a substance or object from solid (Ice) to liquid (water), liquid (water) to vapor (gas), and vice versa when its temperature is constant.
There are mainly two types of latent heat:
- Latent heat of fusion: We denote the latent heat of fusion by ‘Hf’. Latent heat of fusion is the heat energy required to melt a solid (Ice) without changing its temperature. When ice melts, only the phase changes. The temperature remains at 0° C, and the liquid water that forms with the phase change will also be at 0° C.
- Latent heat of vaporization: We denote latent heat of vaporization by ‘Hv‘. Latent heat of vaporization is the heat energy required to vaporize liquid(water) without changing its temperature.
Sensible heat
Sensible heat is the heat energy required to change the temperature of a substance without changing its phase. This heat is the opposite of latent heat where phase changes without changing temperature.
The formula to find sensible heat is Q = mcΔT
Where Q = heat energy
m= mass of substance or object
c=specific heat
ΔT =difference in temperatures (Tf – Ti)
Specific heat
It is denoted by ‘c’. Specific heat is the quantity of heat required to raise the temperature of 1 g of substance by 1 degree Celsius or 1 kelvin.
Calorimetry
Calorimetry measures the heat energy absorbed or released during physical or chemical changes. It involves an instrument calorimeter for monitoring and quantifying the heat exchange.
Principle of calorimetry: When two objects or substances with different temperatures come in contact, the heat transfers from hotter objects to colder objects until they reach thermal equilibrium. Here, the Principle of calorimetry indicates the law of conservation of energy. The total heat lost by an object equals the total heat gained by the other object.
Hess’s law
Hess’s law states that the total enthalpy change for a reaction is the same whether the reaction takes place in one or more than one step.
Mathematically, we express it as ΔHtotal =∑ΔHsteps
For example: Consider we have a reaction: X → Y
If we can break it into two steps:
X → Z(ΔH1)
Z → Y (ΔH2)
Then, according to Hess’s law
ΔHtotal = ΔH1 + ΔH2